Abstract
Introduction
Monosomy 7 (-7) and interstitial deletions of chromosome 7 (7q-) are among the most recurrent chromosomal aberrations found in myeloid neoplasms. Patients carrying these cytogenetical alterations present a poor overall survival (OS), mainly due to a low sensitivity to standard chemotherapy and a high incidence of relapse. In our study, we aimed to disentangle the biology of patients with -7/7q- and find new candidate therapeutic targets for a disease with such a dismal prognosis, by integrating wide genomic approaches.
Methods
We collected bone marrow samples from 487 adult patients at diagnosis, treated in 3 institutions: Institute L. & A. Seragnoli (Italy, n = 213), CEMM (Austria, n = 160), University of Michigan (US, n = 114, GSE23452). Three hundred ninety-five samples were analyzed by SNP arrays (Affymetrix™), 51 samples by mass spectrometry (Metabolon™) and 57 samples by GEP (Affymetrix™) approaches. Chi-squared, fisher's exact test and ANOVA were used to test differences in proportion and distributions. False discovery rate, Bonferroni correction, and Welch's correction were calculated whenever appropriate.
Results
Among the 474 patients with evaluable karyotype, 65 (13.7%) had -7/7q-; 47 (9.9%) had -7, while 18 (3.8%) had 7q-. In our sets, the median age at AML diagnosis was 64 years (21-86) and most of the subjects had a de novo AML (65.1%). WBC count at diagnosis was significantly lower in -7/7q- patients (10.4 vs 35.2 k/mm3 p<0.001). -7/7q- was correlated with secondary and therapy-related AML, (17.5% vs 12.4% and 17.5% vs 3.7%, respectively, p<.004).
Within patients tested for FLT3, NPM1 and TP53 mutation at diagnosis, 1/50 among -7/7q- patients vs 59/300 controls harbored FLT3 ITD mutation (350 patients tested, 2% vs 19.7%, p<0.001). One out of 46 -7/7q- patients vs 62/282 controls harbored NPM1 mutation (328 patients tested, 2.2% vs 22%, p<0.001). Twenty-one out of 40 -7/7q- vs 29/279 harbored TP53 mutation (318 patients tested, 52.5% vs 10.4%, p<.001).
In terms of outcome, -7/7q- AML had a median of overall survival of 10.3 months (95% C.I 5.8-14.8), which accounted for 49.5 months (95% C.I. 40.5-58.4) in other AML cases.
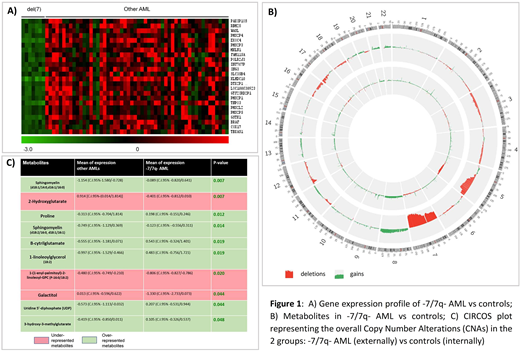
GEP data of a cohort of 57 patients (8 with -7/7q- and 49 controls) revealed that 24 genes were under-expressed in -7/7q- AML (with e-4 significance threshold, Figure 1A). Twenty-three out of 24 genes mapped on chromosome 7; one gene, COX17, mapped on chromosome 3 (Figure 1A). COX17 plays a role in the recruitment of copper to mitochondria.
By metabolomic analyses, we considered quantitative data of 300 different metabolites in 32 patients (4 with -7/7q- vs 28 controls, 19 patients were excluded because samples at diagnosis were not available). In -7/7q- AML, fatty acids (sphingomyelin, 1-linoleoylglycerol), β-cytrilglutamate and the UDP were overrepresented if compared with other AML cases; on the other hand, galactiol and 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC were underrepresented in -7/7q- patients. Furthermore, -7/7q- AML cells seem to accumulate 3-hydroxy-3-methylglutarate and to have lower levels of 2-Hydroxyglutarate (Figure 1C).
With SNP arrays, we considered copy number alterations in 395 patients (52 -7/7q- patients vs 343 controls, Figure 1B). 5q was the most recurrent concurrent deletion, with a minimal common deleted region (MCDR) in q31.3-q33.3. Additionally, 17p (MCDR p11.2 - p13.1), 12p (MCDR p12.3-p13.1), 16q (MCDRs q11.2-q12.1, q21-q22.1 and q24.2-q24.3), 16p (MCDR p11.2) and chromosome 4 (MCDRs q34.1 and q35.2) deletions also co-occurred in -7/7q-, listed per frequencies (Figure 1B). These regions are enriched for genes controlling cell signaling, DNA transcription, post-transcriptional modifications (such as SUMOylation), mRNA splicing and cellular senescence.
Conclusions
SNP, GEP and metabolomic approaches gave new insights on -7/7q- AML biology, identifying 24 new genes differentially expressed in -7/7q-, and 6 MCDR associated with -7/7q- AML. Deletion of chromosome 16q and 4 were never reported in literature associated to AML. Furthermore, for the first time, we described metabolites associated with -7/7q- AML. These data may represent a useful backbone to search for candidate targets in the setting of one of the most aggressive AML subtypes.
Supported by:EHA Non-Clinical Junior Research Fellowship,HARMONY,Fondazione del Monte,FP7-NGS-PTL,AIRC.
*MCF and MO equally contributed
&CP and GS shared the last authorship
Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Kralovics:MyeloPro Diagnostics and Research GmbH: Equity Ownership. Soverini:Incyte Biosciences: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy. Martinelli:Ariad/Incyte: Consultancy; Celgene: Consultancy, Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Amgen: Consultancy; Janssen: Consultancy; Abbvie: Consultancy; Roche: Consultancy; Jazz Pharmaceuticals: Consultancy. Cavo:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal